Technological advances in HPLC protein purification, also known as HPLC peptide purifications, have made the mass manufacture of tailor-made peptides possible in the contemporary period. Developing reliable peptide purification techniques has become more important as their use in scientific research has expanded. Visit our website to find out more about our methods to guarantee that every peptide we sell is purer than 99%. This page will explain the numerous purification steps during peptide synthesis, the various types, processes, techniques, and strategies for purifying peptides, and the various contaminants that may be eliminated by purification.
Because of their complexity, several purification procedures that work well for other organic compounds may not apply to peptides. In order to supply the cleanest peptide to clients at the most affordable price, maximizing efficiency and yield during synthesis is essential. Crystallization-based purification methods work well for certain substances, although peptides are often purified using chromatographic techniques such as high-pressure reversed-phase chromatography.
Removing Certain Contaminants From Peptides
For scientific purposes, the produced peptide must be as pure as possible. Some types of study need a higher minimum acceptable purity (often more than 95%), such as in vitro investigations. In contrast, others, such as using an ELISA standard for detecting titers of antibodies, require a lower minimum acceptable purity (typically more significant than 70%). However, manufacturers must attain enough purity. Recognizing the sources and kinds of impurities is essential for maintaining quality control and avoiding contamination. After that, researchers may use the proper purification method(s).
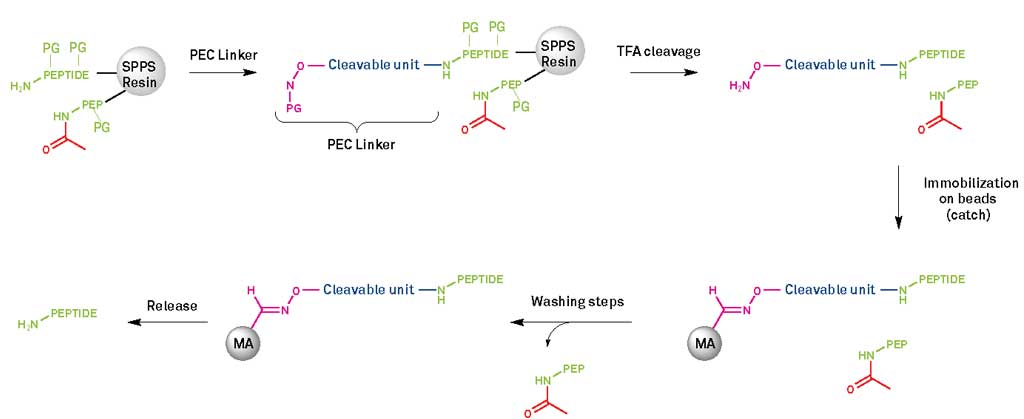
Hydrolysis products of labile amide bonds, deletion sequences created primarily on solid-phase peptide synthesis (SPPS), diastereomers, insertion peptides, and byproducts formed after the removal of protecting groups are all examples of impurities that may arise during peptide synthesis. The last stage of peptide synthesis is susceptible to this later impurity. Additionally, polymeric versions of the synthesized peptide may develop as a consequence, often from the production of cyclic peptides with disulfide linkages.
Peptide purification techniques must be reliable in isolating the desired peptide from a complex mixture, including several other molecules and contaminants.
Strategies for Purifying Peptides
The purification process, ideally, would include as few stages as feasible while yet yielding the desired level of purity. When two or more purification procedures are run in succession, the results are often superior. This statement is especially true when the chromatographic principles used by each purification method are different. For instance, combining ion exchange chromatography with reversed-phase chromatography may provide a pure end product.
Capturing the bulk of the contaminants from the synthesized peptide mixture is often the first step in the purification process. Most of the uncharged and low-molecular-weight contaminants eliminated at this stage are byproducts of the deprotection process at the end of small peptide purification. However, if a greater purity level is desired, a second purification process may be performed to eliminate even more contaminants. Such a second procedure, which may be thought of as a polishing step, is quite proper, mainly based on the complementary chromatographic approach.
The Process of Purifying Peptides
In addition to the essential columns and detectors, HPLC peptide purification systems comprise several other subsystems and modules. These may include systems for buffer preparation, solvent supply, fractionation, data gathering, and so on. The column is the lifeblood of the HPLC protein purification system, and well-considered design choices may make or break the efficacy of the entire operation. The column’s construction materials and compression mechanisms may affect the purification result, which can be either static or dynamic.
Moreover, it is crucial that all peptide purification techniques be executed in line with current Good Manufacturing Practices (cGMP) and that sanitization is treated as a top priority.
Chromatography by Affinities (AC)
This method can efficiently separate peptides by using the binding affinity between a peptide and a specific ligand linked to a chromatographic matrix. The ligand captures the targeted peptide, while the excess is removed by washing. It’s crucial that this bond can be undone. Desorption-friendly conditions are created by either targeted or random manipulation of environmental factors. Desorption may be either selective (using a competitive ligand) or nonspecific (using changes in pH, polarity, or ionic strength). Purified samples of the desired peptide are then accumulated. Using AC may have both a high resolution and a large sample size.
The Use of Ion-Exchange Chromatography (IEX)
The purification method makes use of the fact that different peptides in a mixture have varying degrees of negative and positive charge. Isolating peptides using a chromatographic medium with the opposite charge allows for separating those with a specific charge. The peptides are introduced into the column, where they bond, and the elution parameters are altered to ensure that the bound molecules are released and controlled. Changes are made to the salt content or the pH level of the environment. The combination is often diluted with salt (NaCl). During the binding step, the target peptide becomes more concentrated and may be harvested in its pure form. High resolution and high throughput both describe IEX’s method.
Chromatography Based on Hydrophobic Interaction (HIC)
Hydrophobicity is the underlying premise of this procedure. The desired peptides may be extracted because of the interaction between a peptide and the chromatic medium’s hydrophobic surface. The peptide may be extracted and purified since this connection can be reversed. After an initial purification step using salt in elution, HIC is a very successful purification approach because of the high ionic strength buffer that improves the process (like the IEX technique).
Samples placed onto a column using HIC bond together because of the high ionic strength solution. The next step is elution, which is carried out by lowering the salt content, resulting in the unequal elution of the bound compounds. Ammonium sulfate is often used to dilute the sample along a decreasing gradient since this is a common application technique. Afterward, a concentrated and purified version of the required peptide is gathered. The sampling rate and resolution of HIC are both high quality.
Filtering using Gel (GF)
Gel filtration uses the size difference between the target peptide and the contaminants to achieve peptide isolation. Only samples of low volume make use of GF. A positive aspect of this method is the high quality of the resolution it provides.
Chromatography in Reverse Phase (RPC)
By using the reversible interaction between target molecules and a chromatographic medium’s hydrophobic surface, this purification procedure can extract peptides from impurities at a high resolution. The samples are put onto a column and bound. The next step is to adjust the experimental parameters in a way that results in a selective elution of the bound compounds. Because of the nature of reversed-phase matrices, the initial binding is frequently quite strong, making elution difficult without using organic solvents and other additives. Increasing the concentration of organic solvents, most often acetonitrile is the standard elution method. Pure collections of potent molecules produced by the binding procedure are then obtained. RPC often processes samples of peptides and oligonucleotides as the last polishing step. RPC is not the best purification method if regaining activity and restoring the proper tertiary structure are priorities, as this is often the case with peptides and organic solvents.
Meeting GMP Standards
GMP must be strictly adhered to throughout the whole peptide purification and synthesis operations. This measure guarantees that the final peptide is entirely unadulterated and of the highest possible standard. As part of Good Manufacturing Practices (GMP), all analytical and chemical processes must be meticulously recorded. It is necessary to develop test procedures and requirements ahead of time to guarantee a consistent and repeatable production process.
When it comes to peptide synthesis and purification, BiotechPeptides is as meticulous as it gets. Our commitment to these criteria allows us to provide peptides with a purity greater than 99%, making them ideal for use in any scientific investigation or industrial process.